Hemoglobin
Structure of Hemoglobin Molecule
Hemoglobine (Hb) is a conjugated protein with a molecular weight of 64460. Hemoglobin occurs naturally as a tetramer containing four subunits or monomers, each monomer having molecular weight of 16115. Each monomer consists of a heme part attached to a globin peptide chain. Thus there are 4 heme groups and 4 globin chains in each molecule of Hemoglobin (Hb). Of the 4 globin chains 2 are alpha and 2 are beta chains. In each subunit the combination of heme with globin peptide occurs in the following two ways:
- The two propionic acid groups of heme are attached to the basic groups of lysine and arginine present in globin.
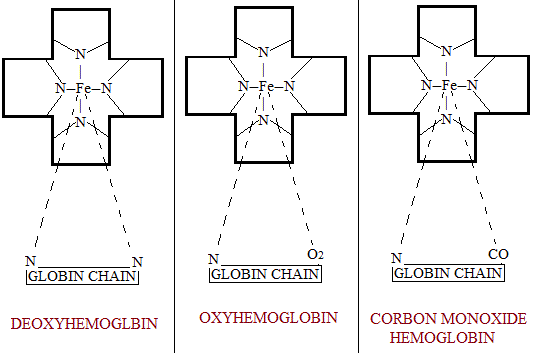
- The iron of heme is attached to 2 imidazole nitrogen of 2 histidine residues of the globin. One of these linkage is quite stable. But the second linkage of the heme iron with the imidazole N is very loose and in the presence of O2 this linkage gets attached to it forming carbon monoxide Hemoglobin. These changes are show in the figures given below. However, there is evidence that this valency of heme iron in case of hydroxyl-Hemoglobin binds a molecule of water instead of being attached to imidazole of globin chain.
Factors Affecting the formation of Hemoglobin
A list of the various factors which affect the formation of hemoglobin and RBCs (erythropoiesis) is given below:
- Metals: These include iron, copper, cobalt and manganese.
- Protein diet.
- Vitamins: The vitamins of special importance for Hemoglobin formation are vitamin B12, folic acid, ascorbic acid, nicotinic acid and pyridoxine.
- Harmones: Anterior pituitary and the thyroid gland take active part in Hemoglobin formation.
- Hypoxia: The stimulant effect of hypoxia on erythropoeisis.
- Erythropoietin: This factor is discussed below in detail
The activity of erythroid marrow has been found to be controlled by a hormonal agent produced in the kidney and called erythropoietic factor. This renal erythropoietic factor (REF) is liberated in response to renal hypoxia. Greater the degree of renal hypoxia, greater is the amount of this erothropoietic factor (REF) secreted. The secretion of the REF is also increased by cobalt and testerone. The REF acts like an enzyme on an α-globin of the plasma from which the active hormone called erythropoietic is liberated.
Erythropoietin is an α-globin and has been obtained in a highly active form. Its activity is found in a plasma glycoprotein with a molecular weight about 60,000. Some erythropoietin activity is also present in the urine. Erythropoietin is considered to act by conversion of certain stem cells to proerythroblasts in the bone marrow with a resultant increase in the number of mature red blood cells. An increased formation of RNA of a high molecular weight is the earliest effect produced by erythropoietin. This is followed by an increase in the RNA of smaller molecular weight (ribosomal, transfer and messenger RNA) and hemoglobin within the immature cell. Patients with increased erythropoietin levels show macrocytosis and increased red cell hemoglobin. Immature reticulocytes are seen in the peripheral blood which are larger and more basophilic than usual. The radioactive iron studies show that the marrow iron transit time which normally is 3.5 days is greatly decreased. That erythropoietin is essential for normal erythropoiesis is shown by the fact that anit-erythropoietin antibodies cause anemia.
The erythropoietic system can respond to hypoxia within 6 to 8 hours. However, it takes 3 to 4 days for the reticulocyte count to rise. The increased erythropoietin formation in hypoxic state is shown by it increased excretion in the urine. Some kidney tumors produce excess of erythropoietin.




