Hemoglobin (Hb) Derivatives
Hemoglobin (Hb) is the chief protein of the red blood cells. A single Red blood cell contains about 280 million molecules of Hemoglobin (Hb). Hb can give rise to many derivative which are discussed below.
 Action of Acids
Action of Acids
Acids split Hb into globin and heme (containing ferrous iron and therefore also called ferroheme). IN the presence of O2 the ferroheme is oxidized to ferriheme which holds the negatively charged acid anion. HCl will act upon Hb in the following way.
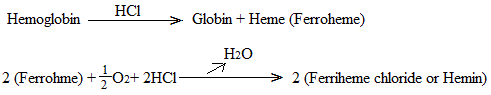
This reaction is basis of Sahli’s method for the determination of Hb of the blood.
 Action of Alkalies
Action of Alkalies
Alkalies also split Hb into globin and ferroheme; the latter is then oxidized to ferriheme which gives rise to alkali hematin on combining with hydroxyl group.
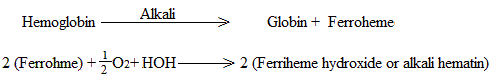
 Reaction with CO2
Reaction with CO2
This leads to the formation of carbamino compounds.
 Reaction with CO
Reaction with CO
This results in the formation of carbon monoxide Hb(CO-Hb). Small amounts of CO are produced endogenously in man as a result of cleavage of the porphyrin rings of Hb molecules during catabolism of the latter. In heavy smokers as much as 5% or more of the blood Hb may be combined with CO. The affinity of Hb for CO is about 210 times more than that for O2. Moreover, CO-Hb is much (about 250 times) more stable as compared to oxy-Hb and thus it does not easily dissociate. The toxic effect of CO-Hb are due to the factors given below:
- CO-Hb is quite useless as carrier of O2 and its formation results in a decreased O2 carrying capacity of the blood.
- The presence of CO-Hb in the blood inhibits the oxygenation of the remaining Hb in the lungs.
- The giving up of O2 by oxy-Hb to the tissues is also inhibited by CO-Hb.
- CO is a powerful enzyme poison; the cytochrome oxidase is particularly inhibited by CO thereby inhibiting cellular respiration. The affinity of Hb for CO can be judged from the fact that when air contains only 0.04% CO, 52% of the Hb becomes converted to CO-Hb; breathing this small amount of CO for nearly 4 hours can kill a man. However, if the same concentration of CO is present in O2 instead of air, only 13% CO-Hb is produced. Thus the presence of a higher concentration of O2 in the breathed air will decrease the formation of CO-Hb and increase the dissociation of CO-Hb already present; this is the basis of the use of pure O2 in the treatment of CO poisoning.
The CO-Hb has cherry red color and the skin and tissues of victims of CO poisoning are tinged with this color.
 Reaction with oxidizing reagents
Reaction with oxidizing reagents
These reagents which include ferricyanide and nitrites cause the production of met-hemoglobin (met-Hb). The formation of met-Hb involves the oxidation of the ferrous iron of hemoglobin to the ferric form. The extra valency of iron serves to bind a hydroxyl or other negative group. Met-Hb is dark brown in color. It can be reduced by Hb by reducing agents such as hydrosulfite.
Under normal circumstances a small fraction of the blood Hb gets converted to met-Hb. However, the red blood cells have a reducing system which in conjugation with glutathione continuously reduces met-Hb to Hb and thus prevents an excessive accumulation of met-Bb. The amount of met-Hb present in human blood is on an average 1.7% of the total blood Hb. The absence of this normal met-Hb reducing system produces congenital methemoglobinemia.
In addition to ferricyanide and nitrites many other chemical agents such as chlorates, peroxides, hydroquinone, pyrogallol, iodine, aniline and sulfonamides can also lead to the formation of met-Hb. Methemoglobinemia occurs mostly in factory workers who work with aromatic nitro or amino compounds or in patient taking sulfonamide and some time it proves fatal. Death is due hypoxia resulting from the following factors.
Met-Hb like CO-Hb can not act as carrier of O2.
The presence of met-Hb also inhibits the dissociation of the remaining oxy-Hb. This aggravates hypoxia still further.
The above mentioned effects of met-Hb lead to cyanosis and dyspnea which are the chief symptoms of methemoglobinemia. Methylene blue and ascorbic acid which are reducing agents are useful in treating methemoglobinemia.
One very important property of met-Hb is its combination with cyanide to form cyanomet-Hb. In the treatment of cyanide poisoning nitrite are administered; this results in the production of methemoglobinemia but the met-Hb so formed takes up cyanide forming cyanomet-Hb. In this way the cyanide ions are not allowed to exert their poisonous effects on the cytochrome oxidase. Later the cyanide is gradually detoxified to thiocyanate and the met-Hb is converted to Hb by the reducing systems present in red blood cells.




