Iron Absorption
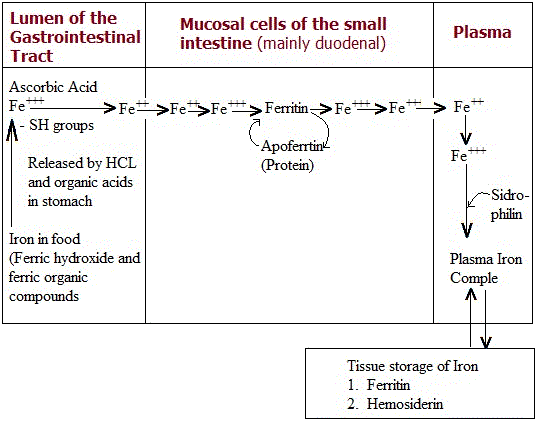
The absorption of iron is quite incomplete and only about 10 % of the iron taken in food is absorbed. In general the mean iron absorption from animal food is twice that from vegetable foods. Most of the food iron occurs in Ferric (Fe+++) state either as ferric hydroxide or as ferric organic compounds. These compounds are broken down into free ferric ions or loosely bound organic iron in the acid medium of the stomach contents where acidity is due to the gastric HCl as well as organic acids of the food. Reducing substances in glutathione convert Fe+++ iron to reduced (Fe++) form. In the Fe++ form iron is more soluble and is more readily absorbed. The gastric HCL is however not essential for iron absorption because achlorchydria iron absorption is not always decreased.
 Mechanism of Iron Absorption (Mucosal Block Regulation)
Mechanism of Iron Absorption (Mucosal Block Regulation)
The intestinal absorption of iron is dependent upon the presence in the intestinal mucosal cells of a protein named apoferritin. It consists of 20 peptide chains and has a molecular weight of 460000. Apoferritin takes up iron by the reaction
Apoferrin + Fe+++ ---------------->Ferritin
This reaction is reversible and ferritin can release its iron. Ferritin is a conjugated protein that contains iron in the form of ferric hydroxide-ferric phosphate complex and its iron content may be upto 23%. Ferritin also occurs in the liver, bone marrow and spleen where it acts as an iron store.
The absorption of iron continues as long as some apoferritin is available in the mucosal cells. When all the apoferritin has been used up (forming ferritin), the absorption of iron stops. Thus non-availability of apoferritin blocks the absorption of iron. The ferritin present in the mucosal cells splits, liberating free apoferritin and iron; apoferritin is utilized again to absorb more iron while the iron goes to the plasma. Any condition leading to an increased apoferritin content in the mucosal cells will result in an increased rate of iron absorption. However, more recent work has shown that the mucosal block to iron is not perfect.
 Plasma Iron Transport
Plasma Iron Transport
Before iron can leave the intestinal mucosal cells, it is first converted to Fe++ form. On the entering the plasma it is again oxidized to Fe+++ form and is taken up by a plasma protein (β-globulin) called siderphillin or transferring, having molecular weight around 90,000. The transfer of iron to the transferring is catalyzed by a Cu-containing protein, namely ceruloplasmin. One molecule of siderophillin binds 2 atoms of iron. The absorbed iron is stored in the body as ferritin in the reticuloendothelial cells of liver, bone marrow and spleen. If iron is in excess, it is also stored as another storage form called hemosiderin (iron content up to 55%). Hemosiderin occurs as microscopically visible granules because it is insoluble due to its containing a characteristic arrangement of the micelles of Fe(OH)3. It is readily demonstrated in tissues by Prussian blue reaction.
A scheme showing iron absorption, transport and storage is given below: