Folic Acid (FA) or Pteroyglutamic Acid (PGA)
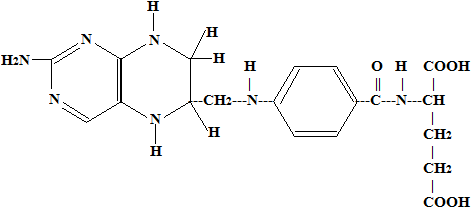
It is a yellow crystalline substance and is only slightly soluble in water. It is heat stable in neutral or alkaline media but not in acid medium. Its structure has 3 components –a derivative of pteridine, para-aminobenzoic acid (PABA) and glumatic acid. There are some other closely related substance which have more than one molecule of glumatic acid in their molecules.
 Occurrence of Folic Acid
Occurrence of Folic Acid
This vitamin is very widely distributed in nature. It is named folic acid because it occurs especially in the foliage of Plants; it has also been called folacin. Modern tendency is to call it by its chemical name i.e. pteroylglumatic acid abbreviated as PGA. Its chief dietary sources are liver, kidney, beef, cauliflower and wheat. Folic acid is also synthesized by intestinal bacteria but this supply is not very significant. It is stored in the liver.
 Biochemical Role of Folic Acid
Biochemical Role of Folic Acid
Folic acid is not active as such but is first reduced to its tetrahydro form represented as FAH4.
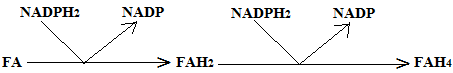
These reactions are catalyzed by the enzyme FA-reductase; ascorbic acid appears to be essential for the reduction of FA to FAH4
FAH4 has the following structure: it is 5,6,7,8, tetrahydro folic acid.

FA.H4 is also inactive as such. But it gives rise to several folic acid coenzymes because it can act as a carrier of various one-carbon groups. These are attached to its molecule in one of the following three ways.
- Attached to Nitrogen No.5
- Attached to Nitrogen No.10
- Balanced between Nitrogen No.5 and No.10
The following scheme shows various one-carbon groups carried by FA.H4 and the mode of their attachment to it.
| One Carbon Group | Mode of Attachement FA.H4 | Name of resulting compound |
|---|---|---|
| (1) --CHO (Formyl) | To N No. 5 | 5-Formyle FA (Folic acid or citrovorum factor) |
| (2) --CHO (Formyl) | To N No. 10 | 10-Formyle FA |
| (3) --CH=NH (Formimino) | To N No. 5 | 5-Formimino FA |
| (4) --CH3 (Methyl) | To N No. 5 | 5-Methyl FA |
| (5) --CH2OH (Hydroxymethyl) | To N No. 5 or 10 | Hydroxymethyl FA |
| (6) --CH2-- (Methylene) | Balanced between N No. 5 & 10 | 5-Methylene FA |
| (7) =CH-- (Methenyl) | Balanced between N No. 5 & 10 | 5-Methylene FA |
These one-carbon groups are utilized in the synthesis of compounds like serine, purines, thymine, methionine, histidine, choline, etc. Thus folic acid coenzyme are involved in the metabolism of nucleic acid, amino acid and phospholipids. A few examples of such reactions are given below:
- Methyl-FA.H4 + Homocysteine ------------> Methionine + FA.H4
This reaction also requires vitamin B12 - Glycine + 10-Hydroxymethyle FA.H4------------> Serine +FA.H4
FA.H4 having a formyl group attached to position No.10 acts as a source of formyl group in the formation of N-formyle methionine-sRNA which initiates the synthesis of peptide chains on ribosomes.
It can be seen that folic acid coenzymes are concerned with a large number of reaction in tissues cells. Its participation in the synthesis of purines and thymine makes it specially important in the growth and reproduction of cells. It is no wonder then that folic acid deficiency is manifested by abnormalities of tissue cells with high mitosis rate such as the cell of the hemopoietic system and those lining the digestive tract.
 Effects of Folic Acid Deficiency
Effects of Folic Acid Deficiency
- Rats show graying of hair and staining of fur and whiskers with porphyrins. Anemia and leucopenia are also seen.
- Human beings show macroytic anemia resembling pernicious anemia except that the nervous involvement of the latter condition is absent. It is accompanied by glossitis and gastrointestinal disturbances.
 Urinary Figlu Test for Folic Acid Deficiency in Human
Urinary Figlu Test for Folic Acid Deficiency in Human
15 grams of histidine monohydrochloride is given orally. The urine is taken after 3 to 8 hours and preserved with glacial acetic acid. It is then subjected to electrophoresis for formiminoglutamic acid (FIGLU). Normally no FIGLU is seen or at the most a trace only. In folic acid deficiency more than 2 mg per hour is excreted and the spot obtained on electrophoresis is well marked. The test is based on the fact that FIGLU is a normal intermediate in the catabolism of histidine; in the absence of folic acid, FIGLU cannot be further broken down in the body and excreted in Urine.
 Clinical Uses of Folic Acid
Clinical Uses of Folic Acid
![]() It is very useful in certain macrocytic anemias, e.g. those associated with sprue, pregnancy, infancy, pellagra and gastric resection.
It is very useful in certain macrocytic anemias, e.g. those associated with sprue, pregnancy, infancy, pellagra and gastric resection.
![]() It can also control the abnormal hemopoiesis of pernicious anemia but has the disadvantage that it cannot check the accompanying degenerative changes in the nervous system. In some cases folic acid administration even hastens the degeneration of the spinal cord.
It can also control the abnormal hemopoiesis of pernicious anemia but has the disadvantage that it cannot check the accompanying degenerative changes in the nervous system. In some cases folic acid administration even hastens the degeneration of the spinal cord.
Folic Acid Antagonists
Drugs like aminopterine and amethopterine (4-amino and 4-amine.N10methyl derivatives of folic acid) interfere with the reaction
As already described, this reaction is essential for the subsequent formation of folic acid coenzymes which are required for the supply of one-carbon compounds. These drugs produce a block in the synthesis of purines and thymine and therefore DNA formation is inhibited. This results in a decreased mitosis especially of rapidly dividing cells, e.g. malignant cells. These drugs are used in the treatment of certain leukemias and certain other conditions associated with an abnormally increased Proliferation of tissue cells. Unfortunately the leukemia cells become resistant to these drugs after some time. Another drug, trimethoprim, inhibits the reaction given above; it is used as an antibacterial and antimalarial drug.




